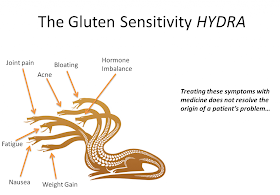
Some report feeling better by going gluten free? Is there a reason behind this? Read on:
"Gluten-free diet: a new strategy for management of painful endometriosis related symptoms?
Marziali M. 1, Venza M. 2, Lazzaro S. 2, Lazzaro A. 2, Micossi C. 2, Stolfi V. M. 2
1 Department of Gynecology and Obsterics, Tor Vergata University, Rome, Italy;
2 Department of General Surgery, Villa Tiberia Hospital, Rome, Italy
2 Department of General Surgery, Villa Tiberia Hospital, Rome, Italy
AIM: Pelvic pain affects 4% to 39% of women and accounts for 10-40% of all outpatient gynecologic visits. The etiology of painful endometriosis-related has not been fully delineated. No studies have been published concerning gluten-free diet administered to achieved relief of painful symptoms endometriosis-related. The aim of this retrospective study was to evaluate the effectiveness for the outcomes of endometriosis-related pain and quality of life of gluten-free diet in a follow-up of 12 months in patients with chronic pelvic pain endometriosis-related.
METHODS: Two hundred seven patients with severe painful endometriosis-related symptoms entered the study. At enrolment time, the baseline values of painful symptoms were assessed by Visual Analogue Scale (VAS) for dysmenorrhoea, non-menstrual pelvic pain, and dyspareunia. According to VAS, pain severity was scored from 0-10; 0 indicating the absence of pain, and 1-4, 5-7 and 8-10 mild, moderate and severe respectively. A gluten-free diet was submitted to all patients and a new evaluation was performed after 12 months of diet. Student t test was used for statistical analysis.
RESULTS: At 12 month follow-up, 156 patients (75%) reported statistically significant change in painful symptoms (P<0.005), 51 patients (25%) reported not improvement of symptoms. No patients reported worsening of pain. A considerable increase of scores for all domains of physical functioning, general health perception, vitality, social functioning, and mental health was observed in all patients (P<0.005).
CONCLUSION: In our experience, painful symptoms of endometriosis decrease after 12 months of gluten free diet.
"Endometriosis is associated with having the HLA-DQ2 and DQ8 genes (which are also present in approximately 96% of patients with Celiac Disease), as well as the DQ7 gene, which has been associated with Celiac Disease in some southern Italians, Sicilians, and Sardinians.
Two studies published within the last few years have shown associations between Celiac Disease and endometriosis. Researchers in Sweden (Stephansson, et al.) reviewed the medical records of over 11,000 women with Celiac Disease in 2011. Compared with controls, women with Celiac Disease were found to be at a much higher risk of having endometriosis, especially in the first year after diagnosis with celiac disease (overall hazard ratio of 1.39). The authors postulate that there must be a shared inflammatory process in both disorders. Likewise, researchers in Brazil found that 2.5% of women diagnosed with endometriosis also had Celiac Disease (Aguiar, et al, 2009). Please see the references section for links to these two studies.
The gluten free diet has recently been recommended as a strategy to manage the pain of endometriosis. In a pilot study in Italy, 75% of women with endometriosis had a decrease in pain symptoms after 12 months on the gluten free diet (see link in reference section). This strongly suggests that gluten sensitivity and/or Celiac Disease plays a role in endometriosis." http://www.celiac.com/gluten-free/blog/1038/entry-1828-celiac-disease-and-endometriosis/
Two studies published within the last few years have shown associations between Celiac Disease and endometriosis. Researchers in Sweden (Stephansson, et al.) reviewed the medical records of over 11,000 women with Celiac Disease in 2011. Compared with controls, women with Celiac Disease were found to be at a much higher risk of having endometriosis, especially in the first year after diagnosis with celiac disease (overall hazard ratio of 1.39). The authors postulate that there must be a shared inflammatory process in both disorders. Likewise, researchers in Brazil found that 2.5% of women diagnosed with endometriosis also had Celiac Disease (Aguiar, et al, 2009). Please see the references section for links to these two studies.
The gluten free diet has recently been recommended as a strategy to manage the pain of endometriosis. In a pilot study in Italy, 75% of women with endometriosis had a decrease in pain symptoms after 12 months on the gluten free diet (see link in reference section). This strongly suggests that gluten sensitivity and/or Celiac Disease plays a role in endometriosis." http://www.celiac.com/gluten-free/blog/1038/entry-1828-celiac-disease-and-endometriosis/
"Gluten is a protein found in glutinous grains (including wheat, rye, barley, spelt, kamut, triticale, graham, bulgur and controversially oats) that many people with endometriosis have difficulty with. In fact, a recent study found that 75% of the endometriosis patients studied had reduced pain while following a gluten-free diet.1 It can be very difficult to digest, leading to increased bowel symptoms, bloating and increased pain. Some people find the problem is specifically wheat and tolerate other glutinous grains just fine. The key to going gluten-free is to choose foods that are naturally gluten-free, like brown and wild rice, quinoa, millet, amaranth, legumes, sweet potatoes and squash instead of processed gluten-free bread, pasta, baked goods, breakfast cereals, bars and crackers which are typically highly refined and fall into the inflammatory category. Also be aware that many condiments contain hidden gluten, so read labels or better still make your own salad dressings, sauces, salsas and marinades to avoid gluten and other inflammatory ingredients." http://www.centerforendometriosiscare.com/nutrition-for-endometriosis/
Addendum:
More on gluten and endo, specifically the association between endo and Celiac's:
"RESULTS:
During the follow-up, 118 individuals with CD and 399 matched controls developed endometriosis. Hence, patients with CD were at increased risk of subsequent endometriosis [HR = 1.39; 95% confidence interval (CI) = 1.14-1.70]. The absolute risk of endometriosis in patients with CD was 112/100,000 person-years with an excess risk of 31/100,000. Risk estimates were highest in the first year after diagnosis (HR = 1.49; 95% CI = 0.83-2.67) and gradually decreased (>5 years after CD diagnosis, HR = 1.33; 95% CI = 1.00-1.79).
"RESULTS:
Nine of the 120 women in the study group were anti-tTGA positive and five of them were also anti-EMA positive. Four of these five patients were submitted to intestinal biopsy which revealed CD in three cases (2.5% prevalence). The overall CD prevalence among the population control group was 1:136 women (0.66%).
Not convinced as this speaks of retrograde menstruation which has been largely refuted as the initial causation of endo but the lab results are interesting to read as is the connections to inflammatory and autoimmune disorders:
"Our study reports a potential association between endometriosis and CD in Italian women, showing a trend for increased prevalence of CD in women affected by endometriosis, even if not statistically significant.
In the last few years, some recent studies about this topic have been performed, showing similar results [17, 18]. The interest arises from shared features of both CD and endometriosis, specifically concerning etiology and ongoing inflammation.
It is well known that CD is an autoimmune disorder in which an abnormal T cell response to gluten occurs. Dieterich et al. recently showed that the tissue enzyme transglutaminase is a target of immunological reaction, generating a complex of the prolamine of gluten with HLA molecule that is recognized by T helper cells [22]. It is noteworthy that CD is strongly associated with some HLA class II genes, in particular with homozygosis for HLA DQ2.5 haplotype; also a condition of heterozygosis for this haplotype associated with the presence of DQ2.2, DQ7, or DQ8 produces a higher risk of CD [23–25].
The presence of gut inflammation, resulting from the above-mentioned immunological response, together with abnormal intestinal permeability and consequent increased antigenic exposure and autoantibody production, could be also responsible for the association of CD with other autoimmune diseases: dermatitis herpetiformis, diabetes mellitus type 1, Sjögren’s syndrome, rheumatoid arthritis, primary biliary cirrhosis, and sclerosing cholangitis. This theory is supported by the evidence that a number of autoantigens, normally cryptics, are processed and presented by APC to T cells, because of prolonged intestinal inflammation [26].
Another factor that could explain the association of CD with other autoimmune diseases is a common genetic background, represented by HLA haplotype; in fact, some genes in the region of major histocompatibility complex are involved in multiple autoimmune disorders, such as HLA DQA1 and DQB1 genes that can confer risk to both CD and type 1 diabetes [27].
Despite decades of extensive research, the pathogenesis of endometriosis remains not completely clarified. Actually, endometriosis is considered a multifactorial disorder, in which the primum movens seems to be represented by retrograde endometrial debris reflux into the peritoneal cavity that promotes increase of oxidative stress and consequent low-grade inflammation [3, 28, 29]. Peritoneal macrophages have been identified as key processes, by producing growth and angiogenic factors, as well as various proinflammatory cytokines that could be responsible for maintenance of disorder and impairment of reproductive function, as well as the systemic involvement that characterized endometriosis [30, 31]. In the last years, chronic inflammation with increased oxidative stress has been reported to be involved also in the association of endometriosis with other chronic inflammatory diseases, as atherosclerosis [32]. According to these evidences, endometriosis is now considered a chronic inflammatory disease, with inflammation not limited to peritoneal cavity but spread to systemic level, as signaled by elevated serum levels of markers as Ca-125 and C-reactive protein (CRP) [33, 34].
Also a genetic predisposition has been suggested for development and progression of endometriosis, with HLA DQ7 haplotype being reported as the first allele significantly associated with endometriosis [35, 36].
A potential role of autoimmunity for endometriosis has been suggested, as it fulfills many of the classification criteria of autoimmune diseases: polyclonal B cell activation, immunological abnormalities in T and B cell functions, defective apoptosis, tissue damage, and multiorgan involvement [37, 38]. This topic is supported also by familial occurrence, genetic predisposition, female preponderance, and increased likelihood of other autoimmune diseases, like systemic lupus erythematosus, rheumatoid arthritis, Sjogren’s syndrome, multiple sclerosis, and endocrine disorders [8, 39].
Among these associations, recently some studies have evaluated the relationship with CD. The first study has determined the presence of CD in a Brazilian subpopulation of women with endometriosis suffering from infertility [17]. The authors have found that prevalence rates of positive CD serology for anti-tTG and antiendomysium IgA in a group of 120 patients with endometriosis versus 1500 controls were statistically significant (4.1% in patients versus 0.8% in controls), even if the prevalence rates of the biopsy-confirmed CD did not reach the statistical significance (2.5% cases versus 0.66% controls), showing only a positive trend, maybe also because one patient refused the endoscopic examination. They have concluded that, even if not statistically significant due to small number of cases, CD is more common in women with endometriosis with respect to controls, suggesting a potential clinical relevance. In the interpretation of their results, however, we cannot ignore that control group was constituted by blood donors; these subjects, as underlined by authors themselves, cannot be considered to be representative of the general population. In fact, some conditions, first of all the presence of anemia (one of the possible manifestations of CD), have to be excluded in subjects candidate as donors. Moreover, in this report total serum IgA assessment was not performed, making it not possible to exclude serum IgA deficiency, a condition that compromises the diagnostic power of serological assays for CD. Finally, since patients with endometriosis were enrolled among subjects referring for infertility disorders, women at higher risk of CD could have been screened, infertility being a complaint also of CD. As regards, in our study the enrollment was conducted among women with endometriosis, not necessarily involving the presence of infertility; however, an increased prevalence of infertility among patients with endometriosis with respect to controls was finally found.
Recently, a Swedish nationwide population-based study has evaluated the risk of developing endometriosis in about 11.000 women with known CD [18]. During the follow-up period of study, the authors have found an increased risk of developing endometriosis, hypothesizing that chronic inflammation characterizing CD could act as trigger in endometriosis development. It is not by chance that they reported that this risk was higher in the first year after the diagnosis of CD, when mucosal healing could not be yet achieved, despite gluten-free diet start. The greater awareness to CD presence, together with improved diagnosis and some socioeconomic factors, has led to increased prevalence of CD, especially identifying mild degrees of CD; pursuant to authors’ view, pointing severity of inflammation due to CD to be crucial for endometriosis development, the presence of mild CD could modify the association with endometriosis. As regards, in our study all subjects diagnosed as affected by CD (both patients and controls), although clinically not suspected for CD, presented villous atrophy with high grades of inflammation at intestinal biopsy.
In conclusion, our results confirm the potential association between CD and endometriosis, although this trend does not reach the statistical significance. Further studies with higher number of subjects are desirable to definitively support this relationship. Actually, we can suggest screening a woman with endometriosis for CD if a valid clinical suspect is present." http://www.hindawi.com/journals/bmri/2014/236821/
Addendum:
More on gluten and endo, specifically the association between endo and Celiac's:
"RESULTS:
During the follow-up, 118 individuals with CD and 399 matched controls developed endometriosis. Hence, patients with CD were at increased risk of subsequent endometriosis [HR = 1.39; 95% confidence interval (CI) = 1.14-1.70]. The absolute risk of endometriosis in patients with CD was 112/100,000 person-years with an excess risk of 31/100,000. Risk estimates were highest in the first year after diagnosis (HR = 1.49; 95% CI = 0.83-2.67) and gradually decreased (>5 years after CD diagnosis, HR = 1.33; 95% CI = 1.00-1.79).
CONCLUSION:
Endometriosis seems to be associated with prior CD. Potential explanations include shared etiological factors and CD-mediated inflammation." http://www.ncbi.nlm.nih.gov/pubmed/21840904"RESULTS:
Nine of the 120 women in the study group were anti-tTGA positive and five of them were also anti-EMA positive. Four of these five patients were submitted to intestinal biopsy which revealed CD in three cases (2.5% prevalence). The overall CD prevalence among the population control group was 1:136 women (0.66%).
CONCLUSION:
This is the first study reporting the prevalence of CD among women with endometriosis, showing that CD is common in this population group (2.5%) and may be clinically relevant." http://www.ncbi.nlm.nih.gov/pubmed/19400413Not convinced as this speaks of retrograde menstruation which has been largely refuted as the initial causation of endo but the lab results are interesting to read as is the connections to inflammatory and autoimmune disorders:
"Our study reports a potential association between endometriosis and CD in Italian women, showing a trend for increased prevalence of CD in women affected by endometriosis, even if not statistically significant.
In the last few years, some recent studies about this topic have been performed, showing similar results [17, 18]. The interest arises from shared features of both CD and endometriosis, specifically concerning etiology and ongoing inflammation.
It is well known that CD is an autoimmune disorder in which an abnormal T cell response to gluten occurs. Dieterich et al. recently showed that the tissue enzyme transglutaminase is a target of immunological reaction, generating a complex of the prolamine of gluten with HLA molecule that is recognized by T helper cells [22]. It is noteworthy that CD is strongly associated with some HLA class II genes, in particular with homozygosis for HLA DQ2.5 haplotype; also a condition of heterozygosis for this haplotype associated with the presence of DQ2.2, DQ7, or DQ8 produces a higher risk of CD [23–25].
The presence of gut inflammation, resulting from the above-mentioned immunological response, together with abnormal intestinal permeability and consequent increased antigenic exposure and autoantibody production, could be also responsible for the association of CD with other autoimmune diseases: dermatitis herpetiformis, diabetes mellitus type 1, Sjögren’s syndrome, rheumatoid arthritis, primary biliary cirrhosis, and sclerosing cholangitis. This theory is supported by the evidence that a number of autoantigens, normally cryptics, are processed and presented by APC to T cells, because of prolonged intestinal inflammation [26].
Another factor that could explain the association of CD with other autoimmune diseases is a common genetic background, represented by HLA haplotype; in fact, some genes in the region of major histocompatibility complex are involved in multiple autoimmune disorders, such as HLA DQA1 and DQB1 genes that can confer risk to both CD and type 1 diabetes [27].
Despite decades of extensive research, the pathogenesis of endometriosis remains not completely clarified. Actually, endometriosis is considered a multifactorial disorder, in which the primum movens seems to be represented by retrograde endometrial debris reflux into the peritoneal cavity that promotes increase of oxidative stress and consequent low-grade inflammation [3, 28, 29]. Peritoneal macrophages have been identified as key processes, by producing growth and angiogenic factors, as well as various proinflammatory cytokines that could be responsible for maintenance of disorder and impairment of reproductive function, as well as the systemic involvement that characterized endometriosis [30, 31]. In the last years, chronic inflammation with increased oxidative stress has been reported to be involved also in the association of endometriosis with other chronic inflammatory diseases, as atherosclerosis [32]. According to these evidences, endometriosis is now considered a chronic inflammatory disease, with inflammation not limited to peritoneal cavity but spread to systemic level, as signaled by elevated serum levels of markers as Ca-125 and C-reactive protein (CRP) [33, 34].
Also a genetic predisposition has been suggested for development and progression of endometriosis, with HLA DQ7 haplotype being reported as the first allele significantly associated with endometriosis [35, 36].
A potential role of autoimmunity for endometriosis has been suggested, as it fulfills many of the classification criteria of autoimmune diseases: polyclonal B cell activation, immunological abnormalities in T and B cell functions, defective apoptosis, tissue damage, and multiorgan involvement [37, 38]. This topic is supported also by familial occurrence, genetic predisposition, female preponderance, and increased likelihood of other autoimmune diseases, like systemic lupus erythematosus, rheumatoid arthritis, Sjogren’s syndrome, multiple sclerosis, and endocrine disorders [8, 39].
Among these associations, recently some studies have evaluated the relationship with CD. The first study has determined the presence of CD in a Brazilian subpopulation of women with endometriosis suffering from infertility [17]. The authors have found that prevalence rates of positive CD serology for anti-tTG and antiendomysium IgA in a group of 120 patients with endometriosis versus 1500 controls were statistically significant (4.1% in patients versus 0.8% in controls), even if the prevalence rates of the biopsy-confirmed CD did not reach the statistical significance (2.5% cases versus 0.66% controls), showing only a positive trend, maybe also because one patient refused the endoscopic examination. They have concluded that, even if not statistically significant due to small number of cases, CD is more common in women with endometriosis with respect to controls, suggesting a potential clinical relevance. In the interpretation of their results, however, we cannot ignore that control group was constituted by blood donors; these subjects, as underlined by authors themselves, cannot be considered to be representative of the general population. In fact, some conditions, first of all the presence of anemia (one of the possible manifestations of CD), have to be excluded in subjects candidate as donors. Moreover, in this report total serum IgA assessment was not performed, making it not possible to exclude serum IgA deficiency, a condition that compromises the diagnostic power of serological assays for CD. Finally, since patients with endometriosis were enrolled among subjects referring for infertility disorders, women at higher risk of CD could have been screened, infertility being a complaint also of CD. As regards, in our study the enrollment was conducted among women with endometriosis, not necessarily involving the presence of infertility; however, an increased prevalence of infertility among patients with endometriosis with respect to controls was finally found.
Recently, a Swedish nationwide population-based study has evaluated the risk of developing endometriosis in about 11.000 women with known CD [18]. During the follow-up period of study, the authors have found an increased risk of developing endometriosis, hypothesizing that chronic inflammation characterizing CD could act as trigger in endometriosis development. It is not by chance that they reported that this risk was higher in the first year after the diagnosis of CD, when mucosal healing could not be yet achieved, despite gluten-free diet start. The greater awareness to CD presence, together with improved diagnosis and some socioeconomic factors, has led to increased prevalence of CD, especially identifying mild degrees of CD; pursuant to authors’ view, pointing severity of inflammation due to CD to be crucial for endometriosis development, the presence of mild CD could modify the association with endometriosis. As regards, in our study all subjects diagnosed as affected by CD (both patients and controls), although clinically not suspected for CD, presented villous atrophy with high grades of inflammation at intestinal biopsy.
In conclusion, our results confirm the potential association between CD and endometriosis, although this trend does not reach the statistical significance. Further studies with higher number of subjects are desirable to definitively support this relationship. Actually, we can suggest screening a woman with endometriosis for CD if a valid clinical suspect is present." http://www.hindawi.com/journals/bmri/2014/236821/